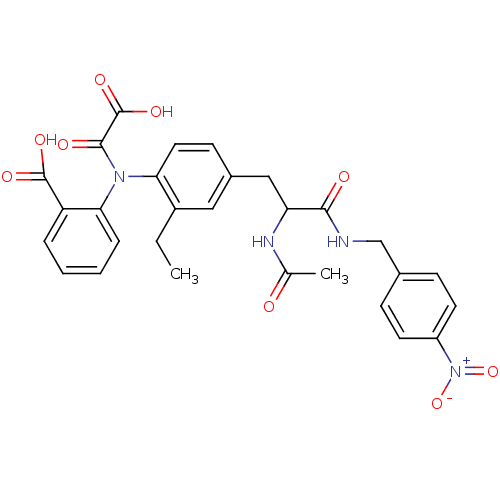
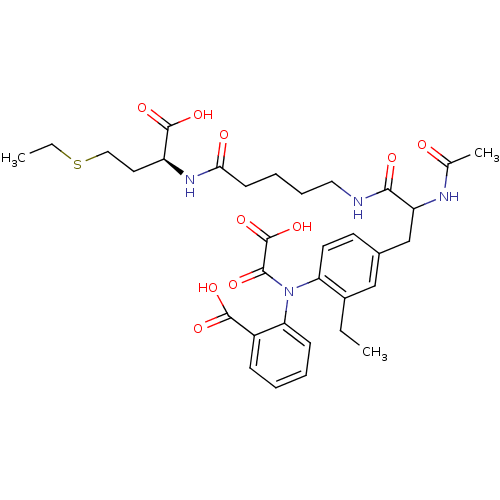
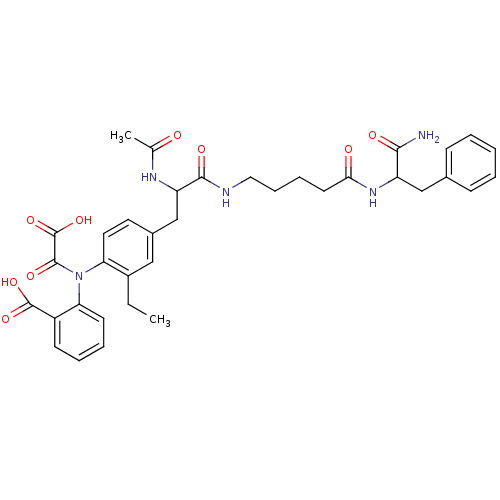
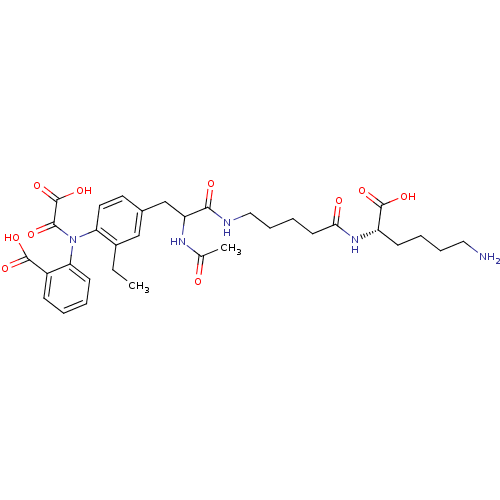
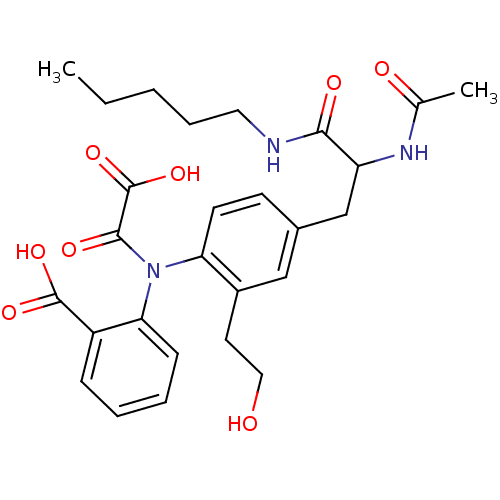
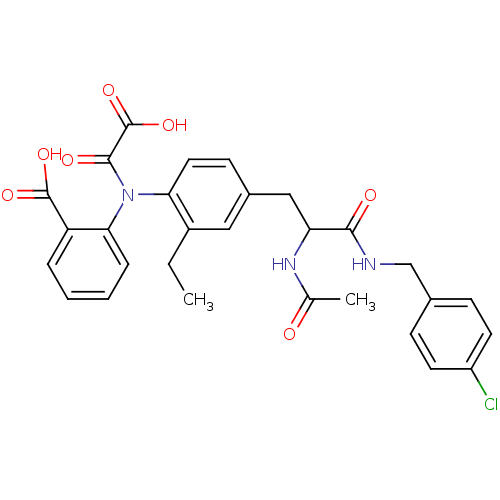
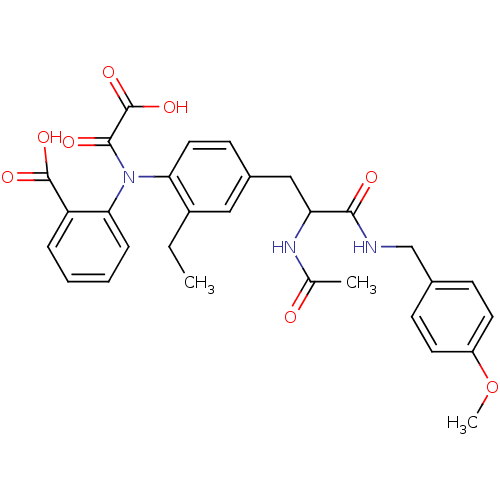
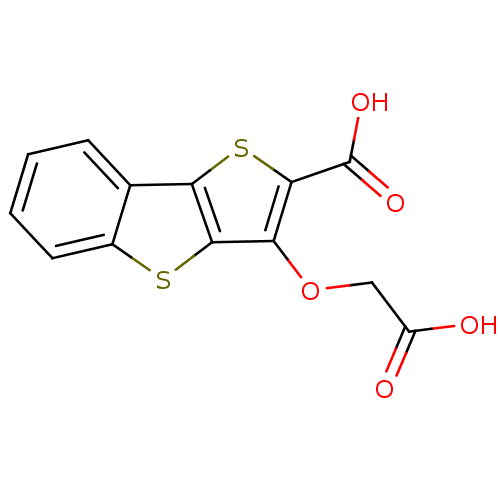
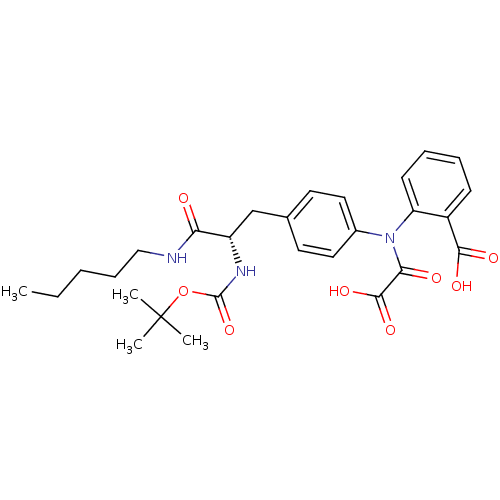
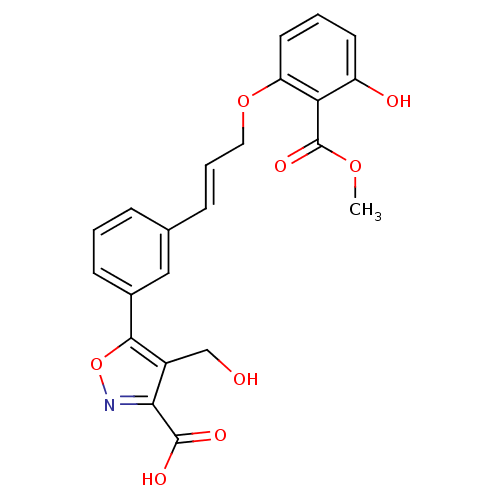
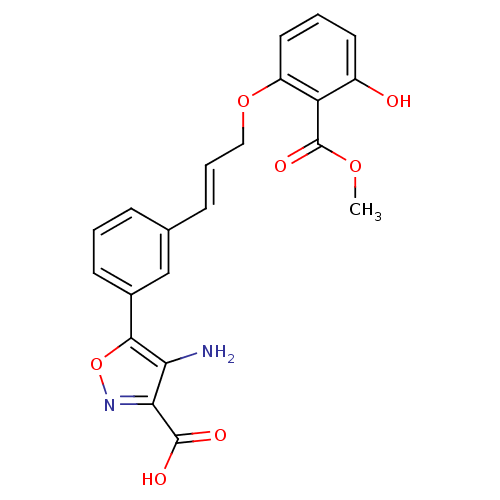
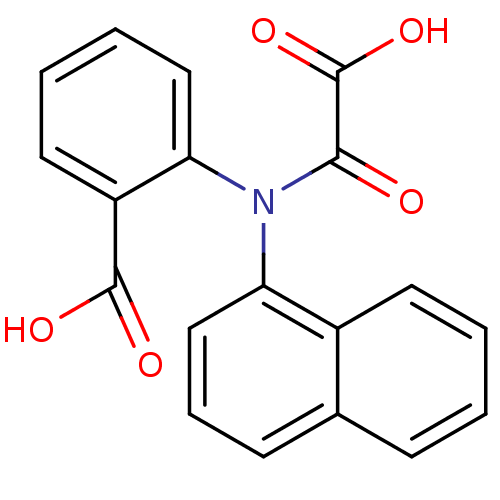
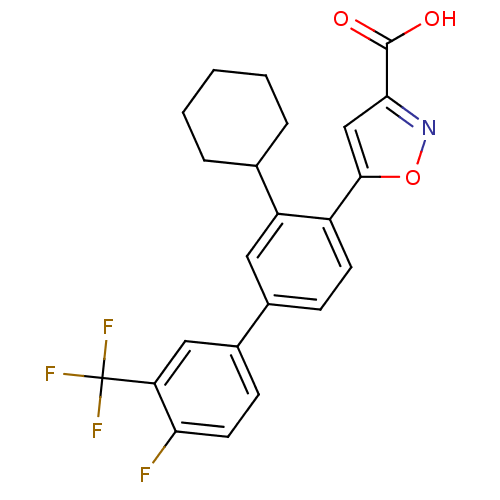
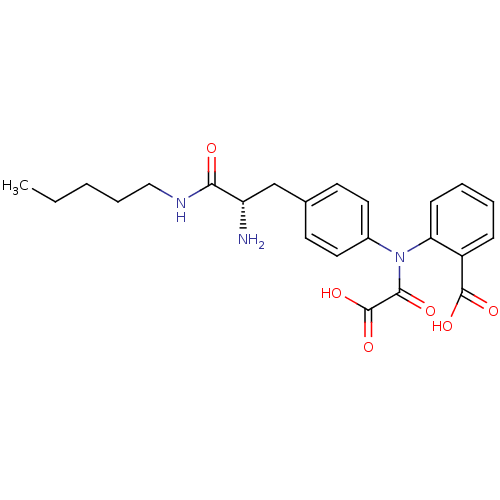
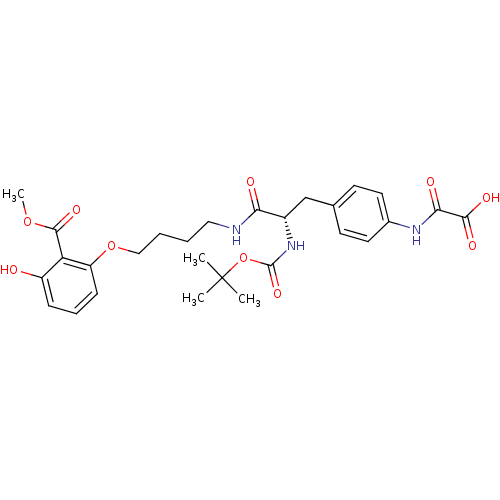
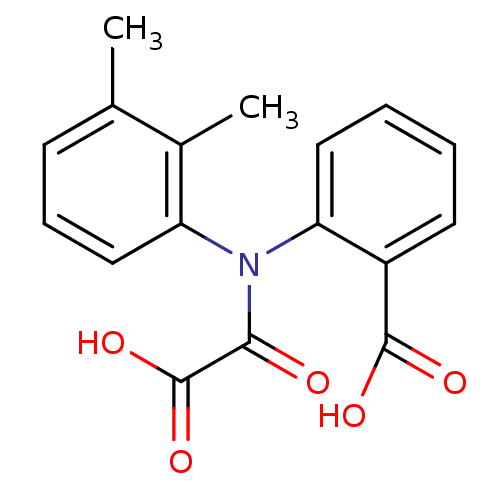
Found 44 of Enz. Inhib. data with enzyme = 'Tyrosine-protein phosphatase non-receptor type 2' and Substrate = 'BDBM13466'
Found 44 of Enz. Inhib. data with enzyme = 'Tyrosine-protein phosphatase non-receptor type 2' and Substrate = 'BDBM13466' Affinity DataKi: 49nM ΔG°: -9.86kcal/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 180nM ΔG°: -9.19kcal/moleT: 2°CAssay Description:The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate...More data for this Ligand-Target Pair
Affinity DataKi: 240nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 270nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 320nM ΔG°: -8.85kcal/moleT: 2°CAssay Description:The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate...More data for this Ligand-Target Pair
Affinity DataKi: 370nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 380nM ΔG°: -8.75kcal/moleT: 2°CAssay Description:The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate...More data for this Ligand-Target Pair
Affinity DataKi: 380nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 450nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 510nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 670nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 680nM ΔG°: -8.41kcal/moleT: 2°CAssay Description:The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate...More data for this Ligand-Target Pair
Affinity DataKi: 700nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 820nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 900nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 900nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nM ΔG°: -8.18kcal/moleT: 2°CAssay Description:The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nM ΔG°: -8.04kcal/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 1.40E+3nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 1.50E+3nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 1.50E+3nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 2.20E+3nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 4.10E+3nM ΔG°: -7.34kcal/moleT: 2°CAssay Description:The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate...More data for this Ligand-Target Pair
Affinity DataKi: 1.09E+4nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 1.30E+4nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 1.92E+4nM ΔG°: -6.37kcal/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: >3.00E+4nM ΔG°: >-6.10kcal/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 4.40E+4nM ΔG°: -5.88kcal/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nM ΔG°: >-5.80kcal/molepH: 7.4 T: 2°CAssay Description:The reaction was started by addition of pNPP substrate, and reaction progress was monitored at 405 nm. The initial rate data collected was used for d...More data for this Ligand-Target Pair
Affinity DataKi: 6.50E+4nM ΔG°: -5.65kcal/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 1.41E+5nM ΔG°: -5.20kcal/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 1.64E+5nM ΔG°: -5.11kcal/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 1.64E+5nM ΔG°: -5.11kcal/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: 1.82E+5nM ΔG°: -5.05kcal/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+5nM ΔG°: >-4.99kcal/molepH: 7.5 T: 2°CAssay Description:The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15...More data for this Ligand-Target Pair